Histiocytoma Complex
Histiocytoma Complex
Histiocytoma
Histiocytomas usually occur as solitary lesions, which usually undergo spontaneous regression. Multiple histiocytomas (i.e. cutaneous LCH) occurs in <1% of cases and are more common in Shar Peis. Recurrence rate for histiocytomas at the surgical excision site are extremely low, and development of a histiocytoma at a new site occurs at extremely low incidence. Metastasis of a solitary histiocytomas to local lymph nodes has been observed. Regression of these lesions is the most common outcome, although systemic spread has also occurred.
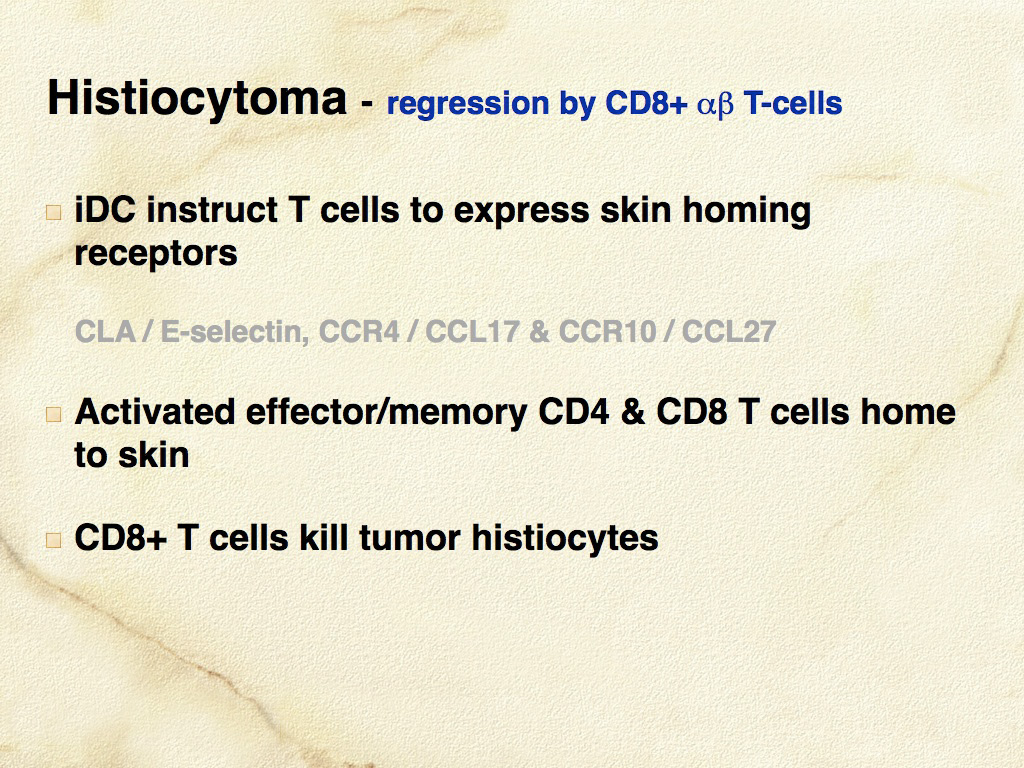
Histiocytomas are progressively infiltrated by lymphocytes and undergo regression after a variable time course. These lymphocytes likely mediate lysis of neoplastic histiocytes, since it has been shown that the tumor infiltrating lymphocytes are highly enriched for CD8+ T cells, which are cytotoxic cells capable of mediating regression. The consistent spontaneous regression of cutaneous histiocytomas is a poorly-understood, fascinating biological phenomenon (see Fig. 1 and 2).
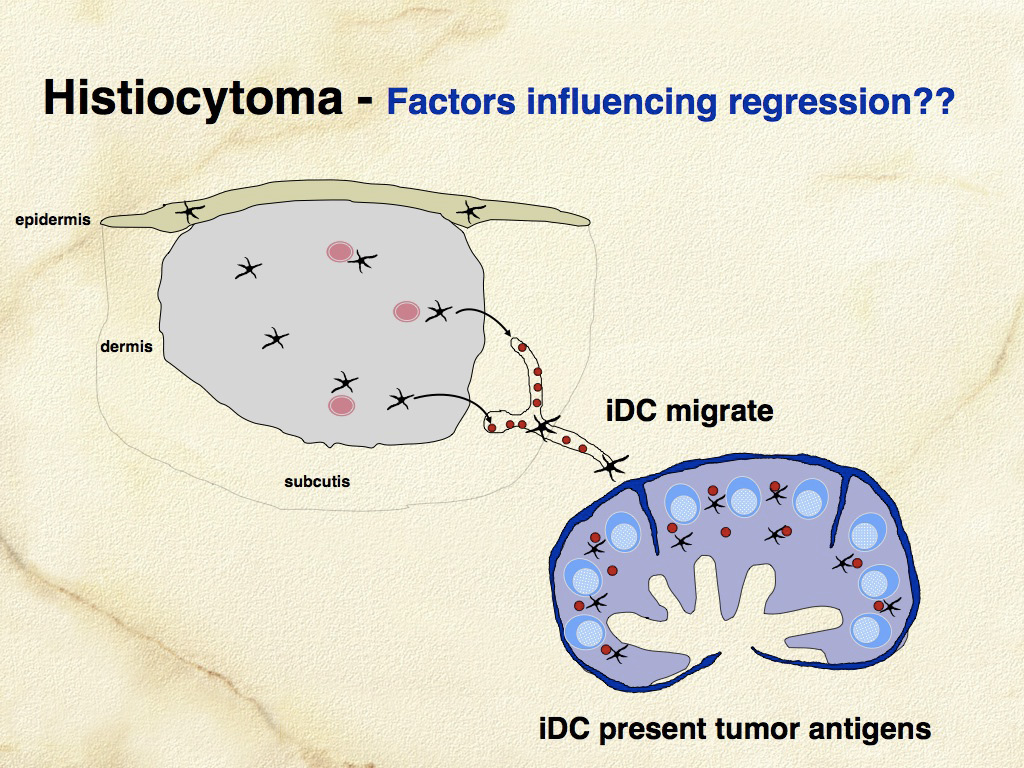
Cutaneous histiocytomas originate in cells that manifest LC differentiation. This is based on immunophenotypic and ultrastructural features. However, Birbeck’s granules have not been conclusively illustrated in normal canine LCs nor in cutaneous histiocytoma. Histiocytomas do exhibit ultrastructural features characteristic of DCs and they also express CD1a and often E-cadherin, which are important functional markers expressed by LCs. Histiocytomas arise in the dermis and may invade the epidermis in a subset of lesions. Hence, histiocytomas do not arise directly from intra-epidermal LCs. It is quite possible that they arise from dermal precursors of LCs similar to the cells described by Larregina and others (Nat Immunol 2001;2(12):1151-1158). These CD14+ dermal cells downregulate CD14, and express CD1a, E-cadherin, and langerin following culture with TGF-β1. They also express CCR6 and are responsive to MIP-3α (CCL20), which is a chemokine produced by keratinocytes that facilitates migration of CCR6+ LCs into epidermis. CD14+ dermal cells did not possess BGs when first isolated, but did develop BGs after culture with TGF-β1. CD14+ LC precursors are found in perivascular locations in superficial and deep dermis. It is not known if an analogous CD14+ LC precursor exists in the dog, but the prospect that cutaneous histiocytoma might originate from a perivascular dermal LC precursor is an attractive hypothesis, which could also explain the stratified expression pattern of E-cadherin within some histiocytomas – stronger expression closer to the epidermis, which is a rich source of TGF-β1.
Morphologic Features
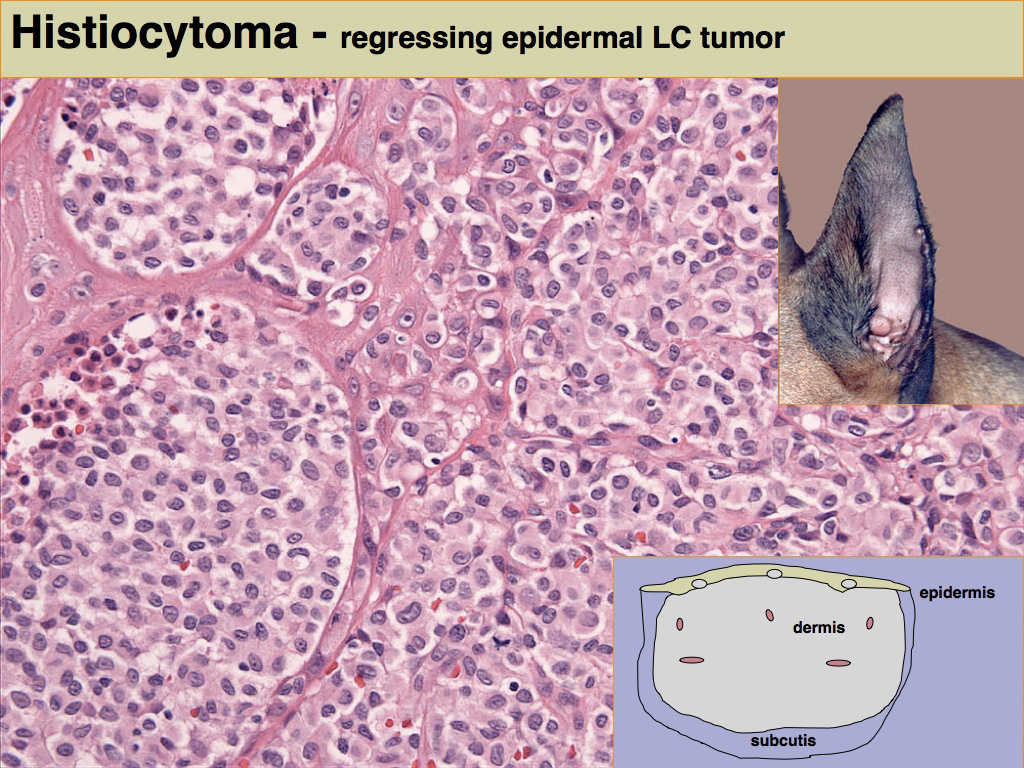
Histiocytomas are most often dome shaped, exophytic growths with complete or partial alopecia; they may attain 4 cm in diameter, but most lesions are less than 2 cm in diameter. Morphological descriptions of histiocytomas emphasize the tropism of the tumor infiltrate for the superficial dermis and epidermis to create a “top-heavy” lesion. In fact tumor histiocytes may invade the epidermis as individual cells or nests of cells (Fig. 3). This can raise concern for epitheliotopic T cell lymphoma. Immunophenotyping using antibodies specific for CD3 and CD18 is necessary to resolve this dilemma.
Tumor histiocytes may display a range of cytological features. Nuclei may be round to oval, indented or complexly folded (convoluted). Multinucleated histiocytes may be observed at low frequency. Cytologic atypia, manifest as anisokaryosis and hyperchromatic nuclei, is unusual. The mitotic index in histiocytomas is highly variable, but is often high. The cytoplasm is usually abundant and eosinophilic. Invasion of the epidermis by individual histiocytes or nests of histiocytes occurred in >60% of lesions in one case study. In a larger context, epidermal invasion is not quite as prevalent as in that report, but it is a diagnostically useful feature and should be carefully evaluated.
Immunophenotypic Features
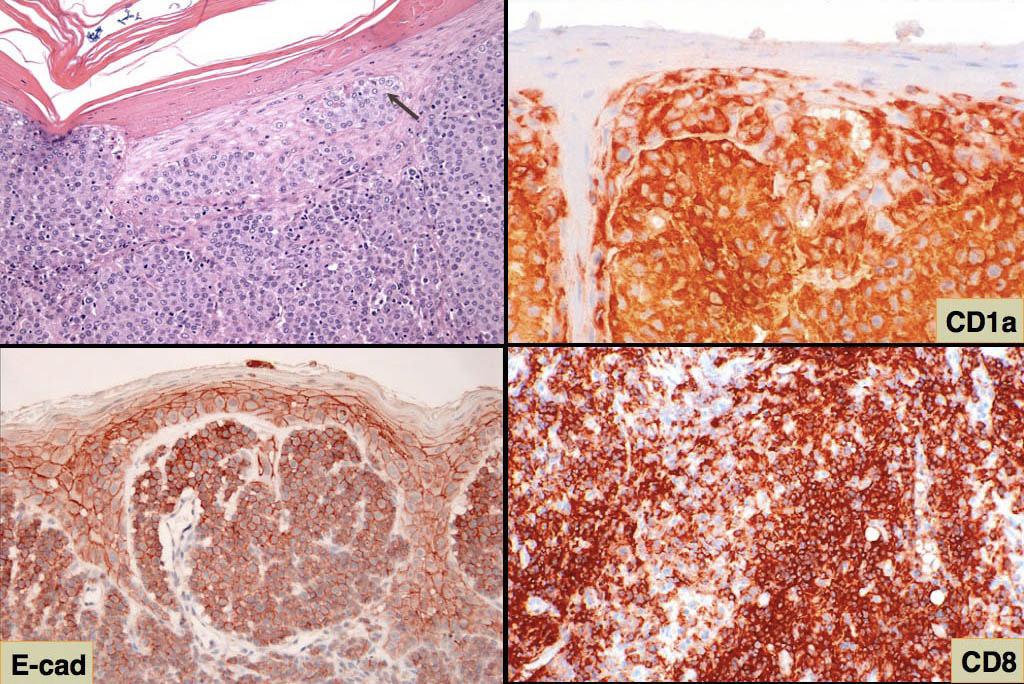
The most comprehensive immunophenotype of canine histiocytomas was established using acetone-fixed sections from snap-frozen tumor tissue (American Journal of Pathology 1996;148(5):1699-1708). The structure of the canine CD1 locus is now largely known based on the most recent canine genome assembly, and the dog has 9 CD1a genes, of which 4 are functional. The CD1 antibodies used in the original 1996 study map to various canine CD1a genes. So in reconciling the results of these 2 papers, tumor histiocytes in canine histiocytoma diffusely expressed CD1a, CD11a/CD18, CD11c/CD18, CD44, CD45 and MHC class II. Tumor histiocytes variably expressed CD11b/CD18 and CD54. They did not express CD90 (Thy-1) and CD45RA. Tumor histiocytes express E-cadherin, which is also expressed by normal LCs (Fig. 4). The true incidence of E-cadherin expression in canine histiocytomas is not known, but it is certainly less than 100% observed in the published reports. Histiocytomas lacking E-cadherin expression occur in our case series (Moore PF, Affolter VK - unpublished data). This could be in part be due to the use of various antibodies to E-cadherin in these studies. It is important to use antibodies that stain the cell membrane where functionally relevant E-cadherin is expressed. Langerhans cells utilize E-cadherin to localize in the epidermis via homotypic interaction with E-cadherin expressed by keratinocytes. E-cadherin is a lineage-associated marker, but not a lineage specific marker for LCs. The staining pattern of E-cadherin in many lesions is not uniform; E-cadherin expression may be limited to the histiocytic infiltrate immediately adjacent to the epidermis. Also, E-cadherin may be expressed by cutaneous round cell tumors of diverse lineage - especially intracellularly but not usually in the cell membrane. Immunophenotyping with multiple markers can clearly resolve this issue, and E-cadherin expression remains a valuable indicator of LC differentiation in cutaneous histiocytoma. Furthermore, differential expression of CD90 and E-cadherin is useful in distinguishing proliferative diseases of interstitial DCs (CD90+) and LCs (E-cadherin+).
Canine cutaneous Langerhans cell histiocytosis
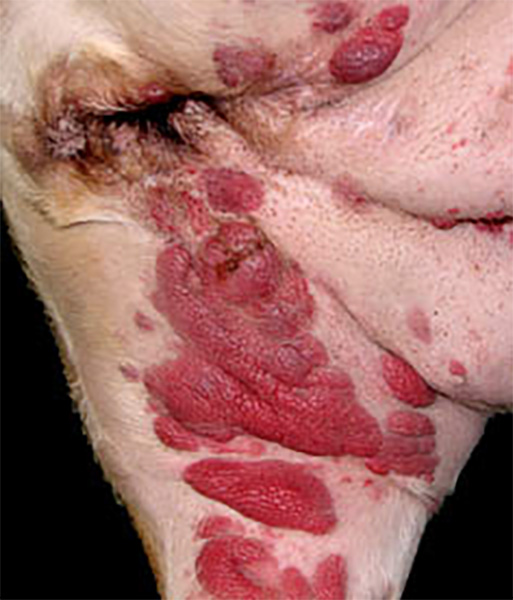
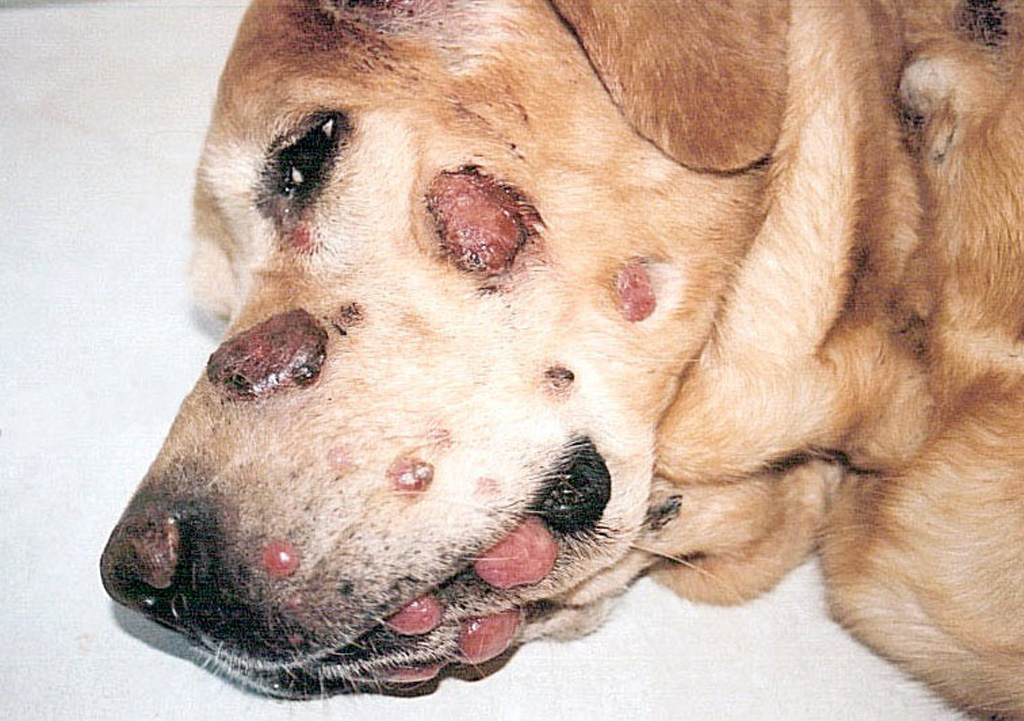
Histiocytomas are almost always solitary lesions. The existence of multiple histiocytomas at the same or distant sites is uncommon. There have been several reports of extensive skin involvement with multiple histiocytomas. This spectrum of disease best fits under the umbrella of cutaneous Langerhans cell histiocytosis (LCH), since skin is invariably involved, and even when systemic disease eventuates, skin is the initial site of origin. Cutaneous LCH (cLCH) is well documented in humans as a single system disease and as a component of systemic disease. Dogs may have literally hundreds of cutaneous lesions ranging from nodules to masses, which elevate the epidermis and may be accompanied by redness, alopecia and ulceration (Fig. 5 and Fig. 6). Lesions at muco-cutaneous junctions and in tissues of the oral cavity may also occur. Lesions may be initially limited to skin, or involve skin and draining lymph nodes. Rarely, internal organ involvement also occurs.
Shar Pei dogs were over represented in a cohort of dogs afflicted by cLCH (about 20% of cases), but the disease occurred in many other breeds. Delayed regression of cLCH is common, and lesions persist for up to 10 months prior to regression. About 50% of the dogs with cLCH were euthanized due to complications in management of the extensive ulcerated lesions and failure of timely regression (Moore PF, Affolter VK - unpublished observations). If cLCH involves lymph nodes, the prognosis is markedly worse. In almost all instances, these dogs were euthanized. The clinical course of dogs that experience systemic spread of cLCH is even more rapid and all dogs were euthanized or died. Necropsies were performed on some dogs. Peripheral lymph nodes and lungs were consistently affected, but metastatic disease can be widespread and involve many other organs (Moore PF, Affolter VK - unpublished observations). This spectrum of disease manifestation and diverse clinical behavior most resembles rapidly progressive multisystem LCH of humans. Consistent treatment strategies were not employed in these cases. Cyclosporine A was used in a number of dogs because the lesions were mistaken for cutaneous histiocytosis; no treatment responses were noted. Nitrogen mustard (CCNU) treatment was effective in most instances, but the responses were not durable. Immune modulators (levamisole) were tried without success (Moore PF, unpublished observations). In one published report, a dog with cLCH was successfully treated with griseofulvin, which is an immune modulating antibiotic. This dog died after developing systemic disease 3 months after griseofulvin was withdrawn.
Morphologic Features
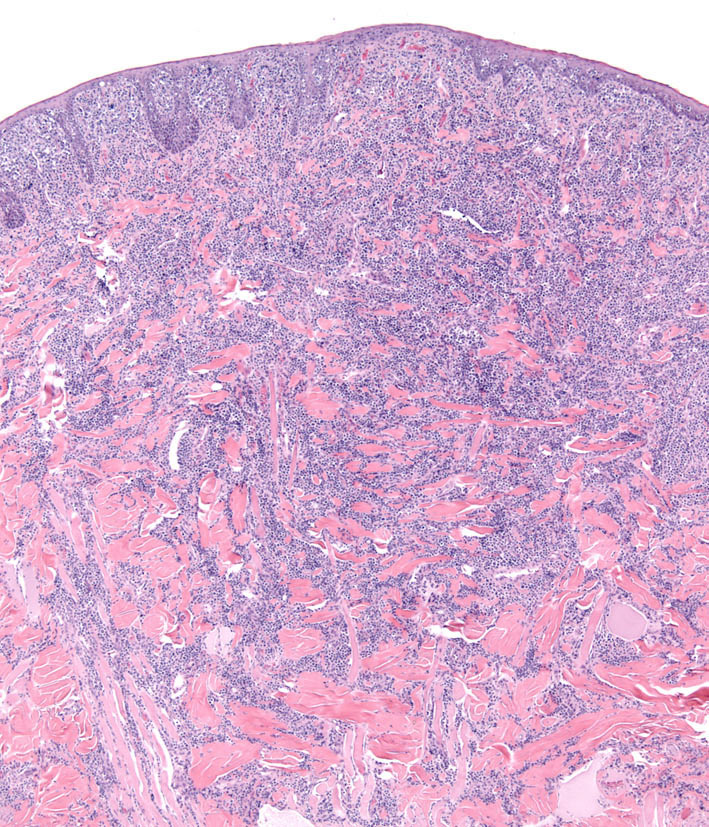
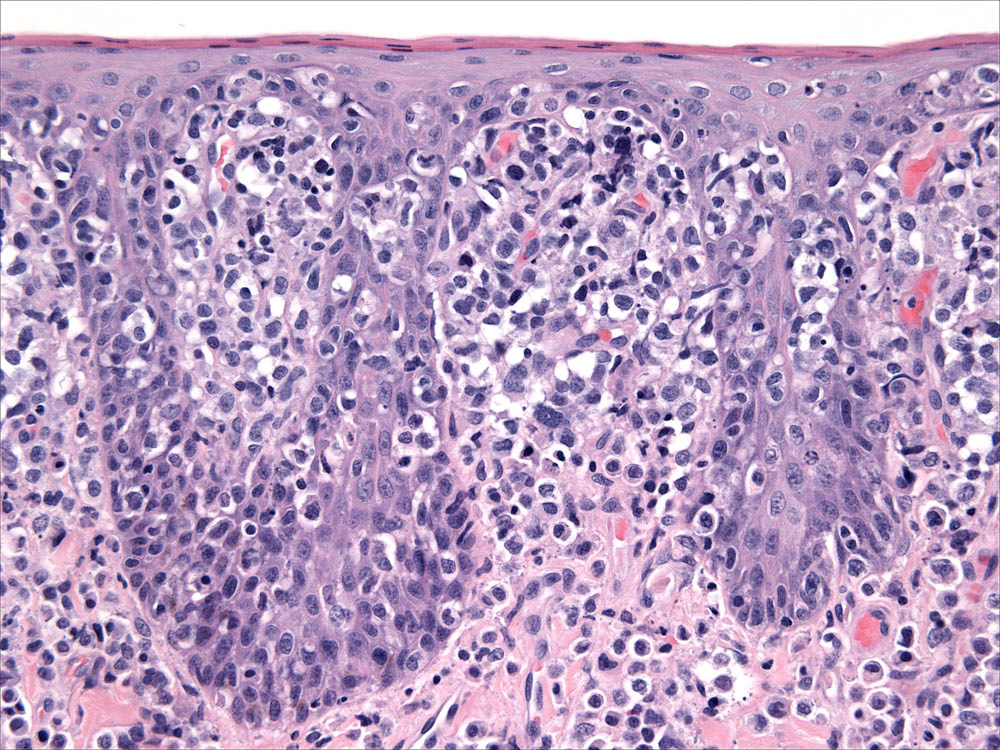
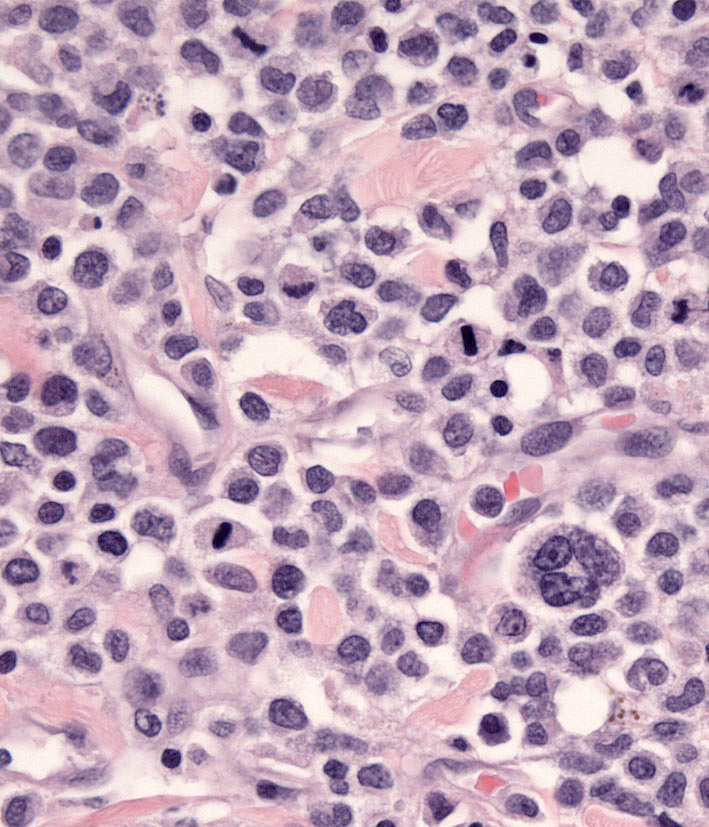
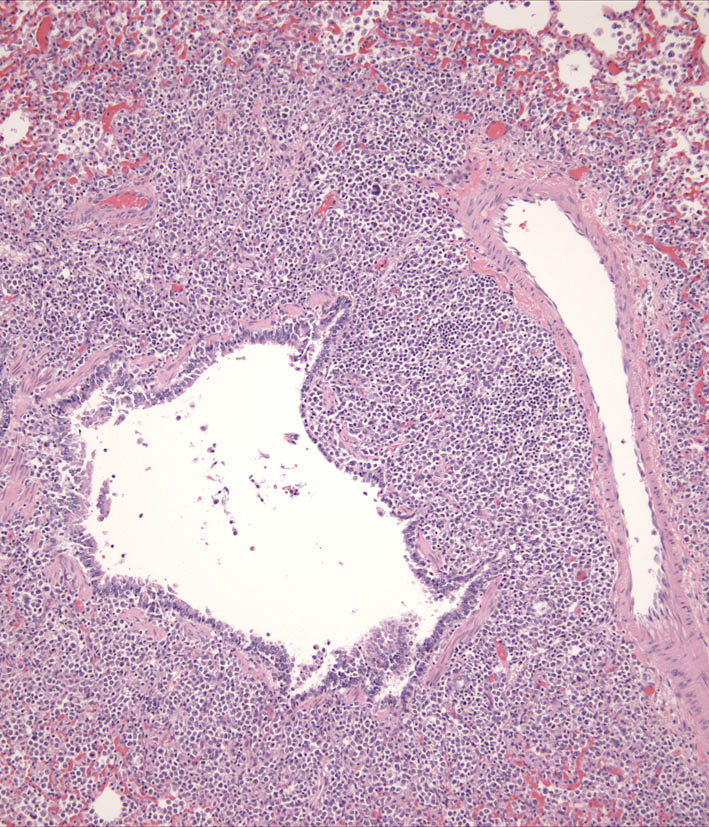
Individual lesions of cLCH often do not differ markedly from those of cutaneous histiocytoma. The lesions are often large masses, which span the dermis and extend into the subcutis and muscle. In aggressive cases, the lesions manifest a higher degree of cytologic atypia, but maintain tropism for the epidermis (Fig. 7 and Fig. 8). Histiocytes may have more anisokaryosis and multinucleated cells than is typical for solitary histiocytoma, but these are not consistent features (Fig. 9). Lymphatic invasion by neoplastic histiocytes portends lymph node involvement, which is an adverse prognostic indicator. Systemic lesions are consistently observed in lung, and the infiltrates have a peribronchial distribution (Fig. 10). Widespread infiltration of other organs is possible.
Immunophenotypic features
Histiocytes in cLCH share the immunophenotype of histiocytes in histiocytoma.
-TOP-
