Histiocytic Lineages
Histiocytic Lineages
The cellular spectrum covered by the term histiocyte has evolved over time. Histiocyte is now considered an overarching term to describe cells of dendritic cell (DC) or macrophage lineage.
Histiocytes differentiate from CD34+ stem cell precursors into macrophages and several DC lineages, which encompass Langerhans cells (LCs) and interstitial DCs. Langerhans cells occur within epithelia of the skin, alimentary, respiratory and reproductive tracts. Interstitial DCs occur in perivascular locations in many organs except the brain, although they do occur in the meninges and choroid plexus. Amongst the most studied DCs are those that occur in skin; these include epidermal LCs and dermal DCs, which are interstitial DCs. Dendritic cells that occur in T cell domains in peripheral lymphoid organs (lymph node and spleen) are known as interdigitating DCs. Interdigitating DCs in lymph nodes are comprised of resident DCs and migratory DCs. The migratory DCs arrive in lymphatics from tissues and consist of LCs and interstitial DCs. The majority of canine and feline histiocytic diseases involve proliferations of cells of LC or interstitial DC lineage; hemophagocytic histiocytic sarcoma (HS) is currently the only histiocytic disease of dogs and cats that has been shown to originate in macrophages.
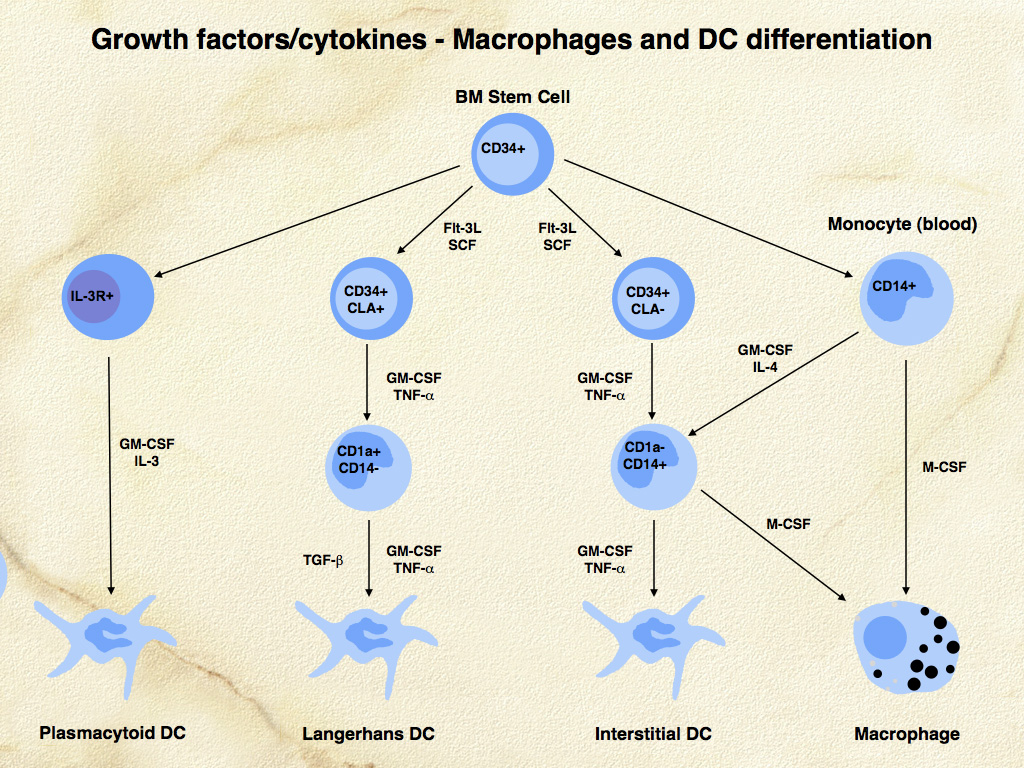
Cytokines and growth factors that influence DC development include fms-like tyrosine kinase (FLT3) ligand, granulocyte-macrophage colony stimulating factor (GM-CSF), stem cell factor, tumor necrosis factor alpha (TNF-α), interleukin 4 (IL-4) and transforming growth factor beta 1 (TGF-β1). Macrophage development from CD34+ precursors is influenced by GM-CSF and macrophage colony stimulating factor (M-CSF). Blood monocytes can differentiate into either macrophages under influence of M-CSF, or into DCs under the influence of GM-CSF and IL-4. Langerhans cell differentiation is critically dependent on TGF-β1 stimulation. Epidermal keratinocytes constitutively secrete TGF-β1, which promotes the differentiation of LCs from dermal CD14+ precursor cells (Fig. 1). These precursor cells up-regulate C-C chemokine receptor 6 (CCR6) and migrate into the epidermis to become immature epidermal-resident LCs in response to macrophage inflammatory protein 3 alpha (MIP-3α or CCL20), which is produced there. Culture of LCs in the presence of GM-CSF and IL-4 results in further differentiation of LCs into functionally mature DCs with enhanced potential to present antigen to T cells with appropriate co-stimulation. These latter conditions are present in states of cutaneous inflammation.
-TOP-
Immunophenotypic markers of histiocytic lineages
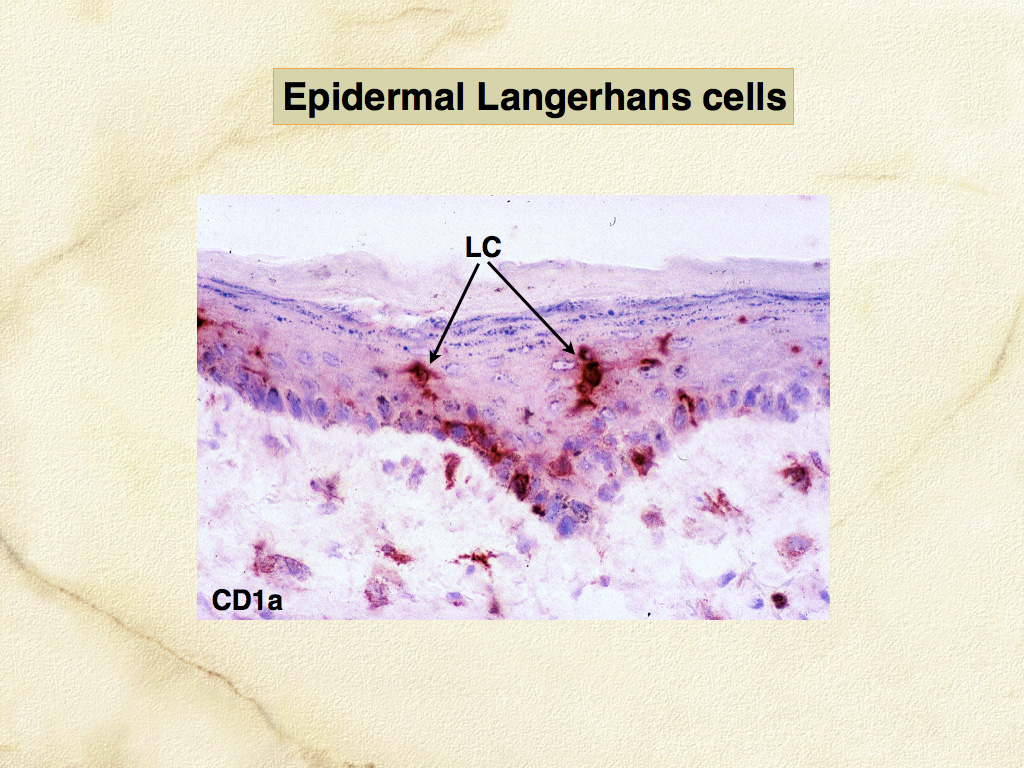
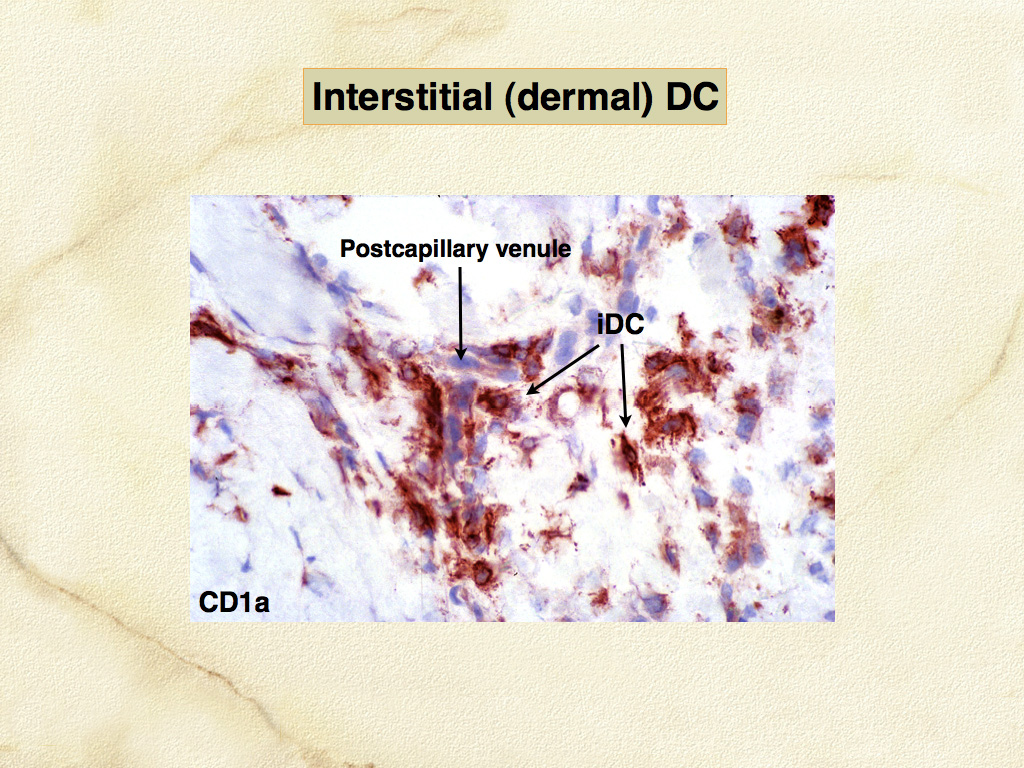
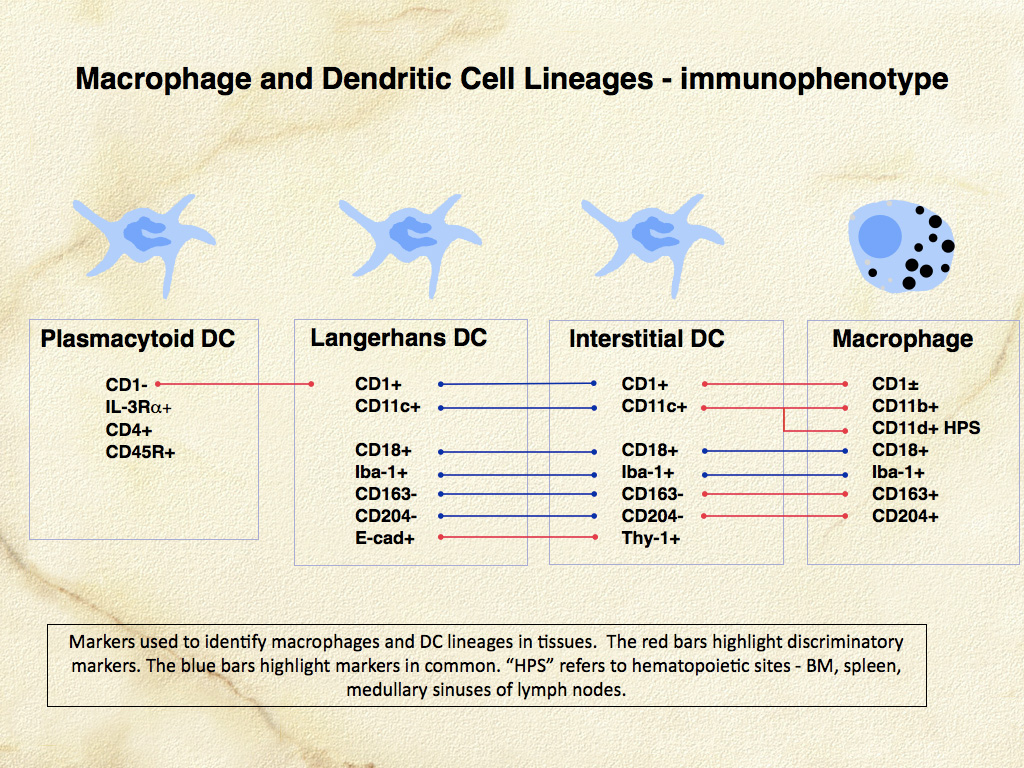
Dendritic cells are the most potent antigen-presenting cells (APC) for induction of immune responses in naïve T cells. The development of canine and feline specific monoclonal antibodies for functionally important molecules of DCs and macrophages has enabled their identification in canine and feline tissues, especially skin. Dendritic cells occur in 2 major locations: within the epidermis (LCs), and within the dermis especially adjacent to postcapillary venules (dermal interstitial DCs) (Fig. 2, 3). Canine and feline DCs express CD1a molecules, which together with major histocompatibility complex (MHC) class I and class II molecules, are responsible for presentation of peptides, lipids and glycolipids to T cells (Fig. 4). Hence, DCs are best defined by their abundant expression of molecules essential to their function as APC. Of these, the family of CD1 proteins is largely restricted in expression to DCs in skin; while MHC class I and II are more broadly expressed.
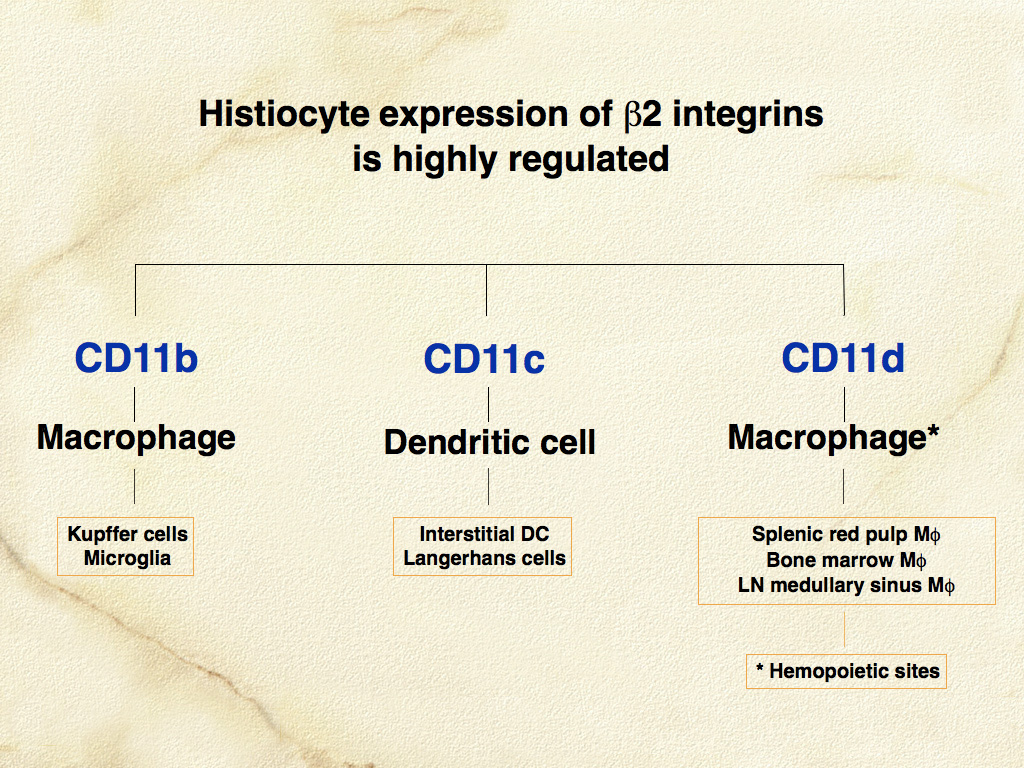
The β-2 integrins (CD11/CD18) are critically important adhesion molecules, which are differentially expressed by all leukocytes. β-2 integrins are hetero-dimeric molecules consisting of one of four α subunits (CD11a-d) and a single β subunit (CD18 or β-2) (Fig. 5). The major hypothesis underlying the development of monoclonal antibodies specific for canine CD11/CD18 was that β-2 integrins would be differentially expressed in canine histiocytic diseases. Canine specific monoclonal antibodies for the known human β-2 integrin α subunits CD11a (αL), CD11b (αM), and CD11c (αX) were produced (Leukocyte Antigen Biology Laboratory, UC Davis - LABL). Also, a monoclonal antibody specific for a novel canine β-2 integrin α subunit was discovered. This antibody was then used to characterize the canine protein, which led directly to the discovery of the human analogue designated αD and by inference CD11d. Subsequently, CD11d expression in canine histiocytic disease was associated with macrophage differentiation in hemophagocytic histiocytic sarcoma, which differed markedly from histiocytic sarcomas of interstitial DC origin; these expressed CD11c. These results support the original hypothesis.
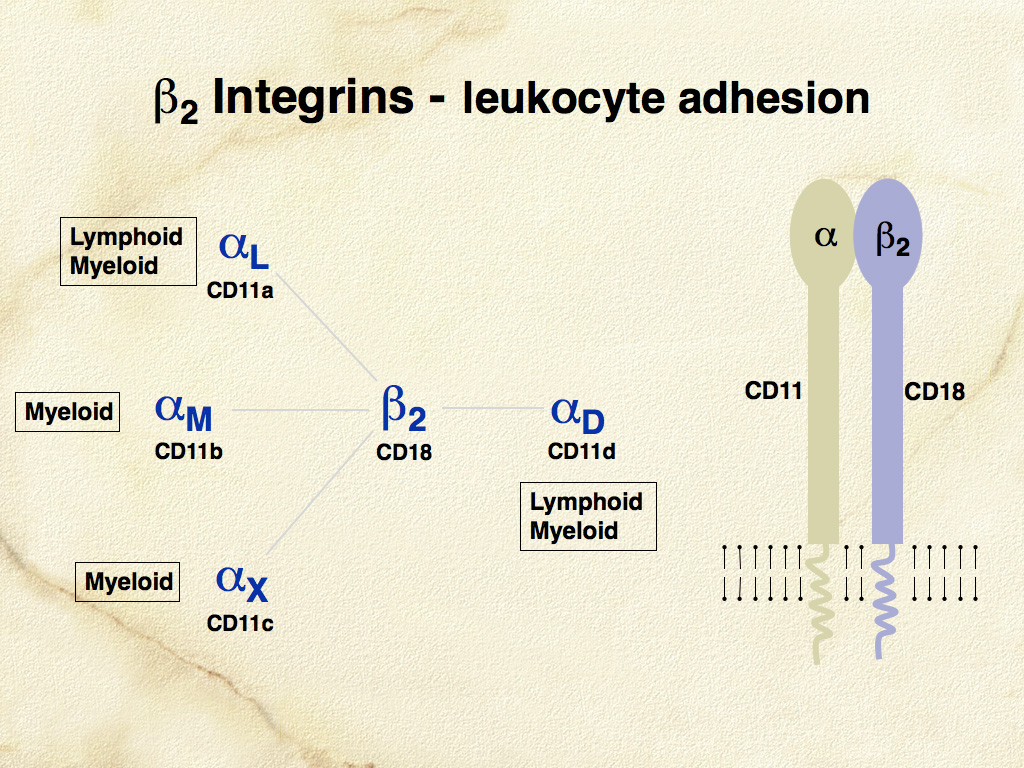
CD11/CD18 expression is highly regulated in normal canine macrophages and DCs. CD11c is expressed by LCs and interstitial DCs; while macrophages predominately express CD11b (or CD11d in hematopoietic environments such as the splenic red pulp and bone marrow) (Fig. 6). A subset of dermal interstitial DCs also expresses CD11b. In diseased tissues these β-2 integrin expression patterns may be broadened. β-2 integrins are detectable in feline tissues. However, monoclonal antibodies specific for feline CD11a and CD11c are not available. Feline CD11b and CD11d are detectable with cross- reactive anti-canine monoclonal antibodies (LABL). Canine and feline CD18 are detectable by species-specific monoclonal antibodies that were developed for use in formalin-fixed tissue (LABL). These monoclonal antibodies have greatly facilitated the investigation of histiocytic diseases in dogs and cats.
Macrophages express an array of scavenger receptors including the class A scavenger receptors, CD163 and CD204. The latter is more broadly expressed by macrophages. Dendritic cells in normal tissues do not express CD163 and CD204, although they are able to upregulate these receptors in diseased tissues to some extent. Langerhans cells and dermal interstitial DCs are also distinguishable by their differential expression of E-cadherin (LCs+) and Thy-1 (CD90) (dermal interstitial DCs+) (Fig. 7). Langerhans cells localize within epithelia via E-cadherin homotypic adhesion with E-cadherin expressed by epithelial cells. Langerhans cells, in the quiescent conditions that prevail in normal skin, are subject to slow turnover and are maintained by self-renewal or renewal from a dedicated dermal precursor cell. Dermal interstitial DCs are subject to more rapid turnover and are repopulated by a blood-borne precursor (perhaps the monocyte) and by local self-renewal.
-TOP-
Dendritic cell migration
Migration of DCs (as veiled cells) beyond the skin to the paracortex of lymph nodes, where they join forces with interdigitating DCs, occurs in the normal steady state, and is enhanced in inflammatory states (Fig. 8). Successful interaction of DCs and T cells in response to antigenic challenge also involves the orderly appearance of co-stimulatory molecules (B7 family – CD80 and CD86) on DCs, and their ligands - CD28 and cytotoxic T-lymphocyte antigen 4 (CTLA-4) on T cells. In-situ DCs have low expression of MHC-II and co-stimulatory molecules and are more receptive to antigen uptake. Migratory DCs upregulate MHC class II and B7 family members and become more adept at antigen presentation to T cells.
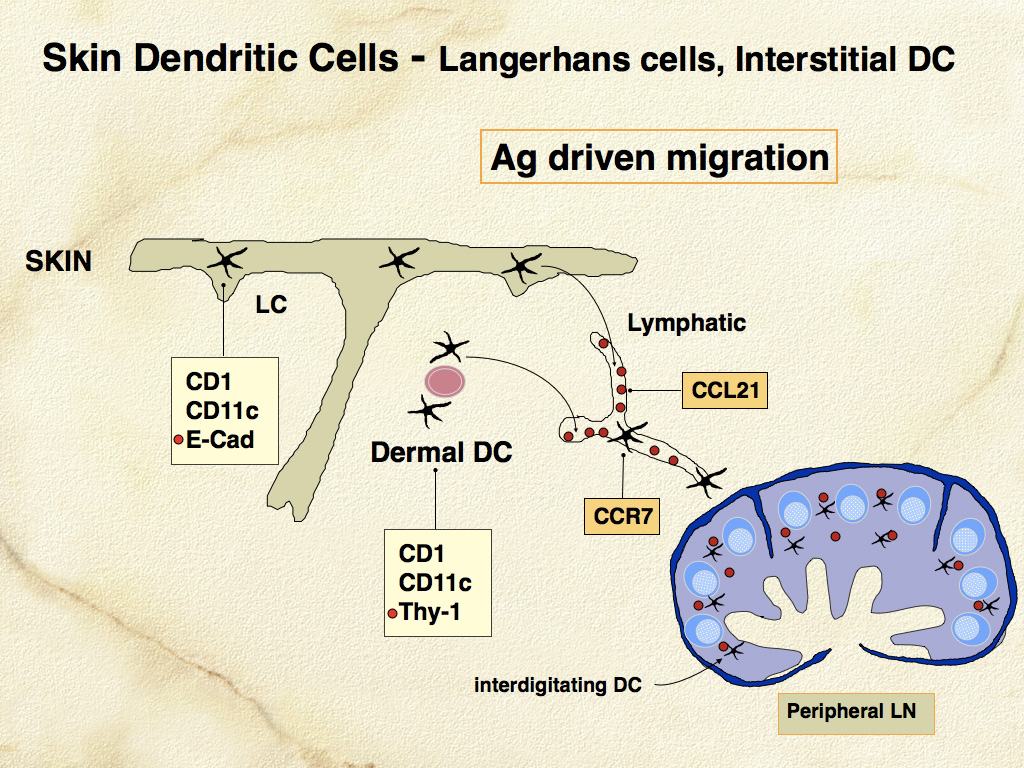
Aspects of the developmental and migratory program of DCs are recapitulated in canine and feline histiocytic diseases. Defective interaction of DCs and T cells may contribute to the development of canine reactive histiocytoses - cutaneous (CH) and systemic histiocytosis (SH), which are related interstitial DC disorders that likely occur in the context of disordered immune regulation. The distant migratory potential of DCs is of immense clinical significance in the adverse prognosis of histiocytic sarcomas, which largely originate in interstitial DCs and rapidly disseminate via metastasis.
-TOP-
